
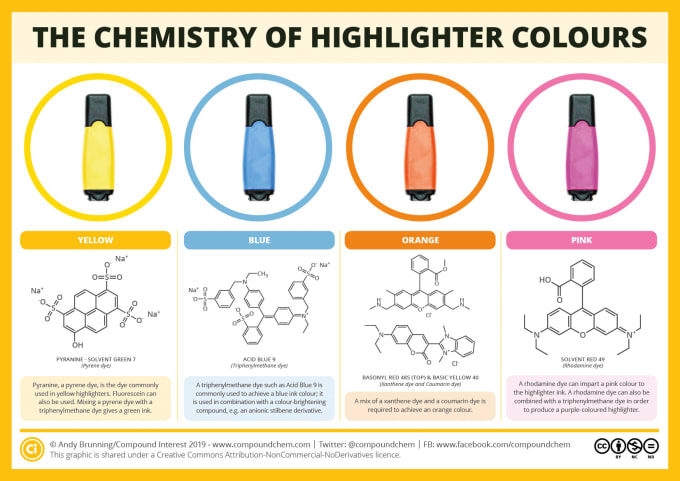
Equilibrium molecular geometry - Locates the nearest local minimum and provides energy and associated properties.If quantum chemical models are employed, the wave function is calculated. Energy – For a given geometry, provides energy and associated properties of a molecule or system.A calculation dialogue provides access to the following computational tasks: Quantum chemistry composite methods, thermochemical recipes.Īvailable computational models provide molecular, thermodynamic, QSAR, atomic, graphical, and spectral properties.Configuration interaction: CIS, CIS(D), QCIS(D), quadratic configuration interaction (QCISD(T)), RI-CIS(D).Time-dependent density functional theory (TDDFT).Head-Gordon group functionals: ωB97, ωB97X, ωB97X-D.Combination or hybrid functionals: B3PW91, B3LYP, B3LYP5, EDF1, EDF2, BMK.Correlation functionals: VWN, LYP, PW91, P86, PZ81, PBE.Exchange functionals: HF, Slater-Dirac, Becke88, Gill96, GG99, B(EDF1), PW91.
The calculated T1 heat of formation (y axis) relative to the experimental heat of formation (x axis) for a set of >1800 diverse organic molecules from the NIST thermochemical database with mean absolute and RMS errors of 8.5 and 11.5 kJ/mol, respectively. Spartan applies computational chemistry methods (theoretical models) to many standard tasks that provide calculated data applicable to the determination of molecular shape conformation, structure (equilibrium and transition state geometry), NMR, IR, Raman, and UV-visible spectra, molecular (and atomic) properties, reactivity, and selectivity. Quantum chemical calculations can supply information to complement existing experimental data or replace it altogether, for example, atomic charges for quantitative structure-activity relationship (QSAR) analyses, and intermolecular potentials for molecular mechanics and molecular dynamics calculations. Quantitative calculations, leading directly to information about the geometries of transition states, and about reaction mechanisms in general, are increasingly common, while qualitative models are still needed for systems that are too large to be subjected to more rigorous treatments. Quantum chemical calculations are also called upon to furnish information about mechanisms and product distributions of chemical reactions, either directly by calculations on transition states, or based on Hammond's postulate, by modeling the steric and electronic demands of the reactants.

Quantum chemical calculations, including Hartree–Fock method molecular orbital calculations, but especially calculations that include electronic correlation, are more time-consuming in comparison. Molecular mechanics calculations on complex molecules are common in the chemical community. Primary functions are to supply information about structures, relative stabilities and other properties of isolated molecules. Quantum chemistry calculations in Spartan are powered by Q-Chem.
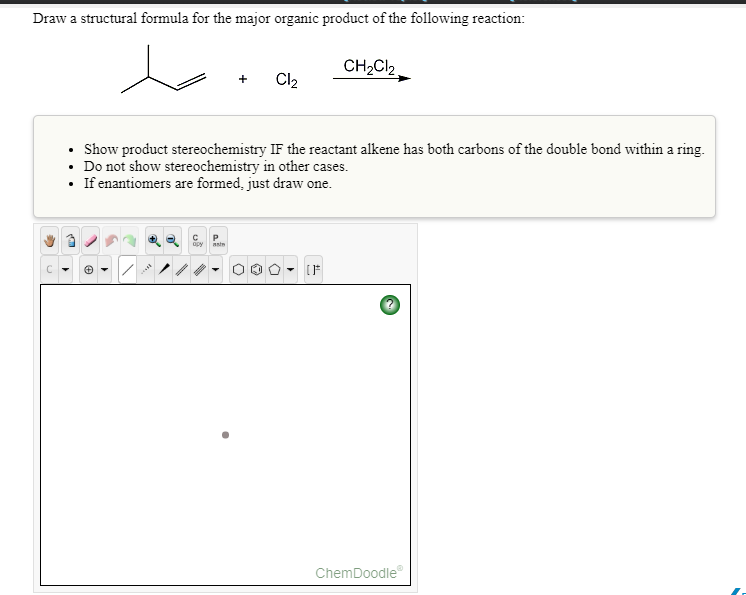
#CHEMDOODLE CITATIOON CODE#
It contains code for molecular mechanics, semi-empirical methods, ab initio models, density functional models, post-Hartree–Fock models, and thermochemical recipes including G3(MP2) and T1. Spartan is a molecular modelling and computational chemistry application from Wavefunction. Molecular modelling, computational chemistry


 0 kommentar(er)
0 kommentar(er)
